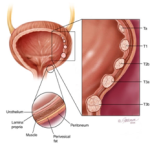
What is Intravesical Immunotherapy?
It is a form of treatment where specific medicine is introduced into the urinary bladder in patients who are diagnosed with cancer of the urinary bladder.
What is the goal of this therapy?
The goal is to decrease recurrence of the disease, prevent progression and eradicate residual disease after endoscopic surgery for the cancer.
What medicine is used?
Most commonly used is BCG (Bacille Calmette – Guerin). It is a live, but weakened strain of tuberculosis bacteria.
When is this treatment started?
This is usually started after 2 to 4 weeks of your initial endoscopic operation.
Do I need to have any special precautions prior to coming to the hospital for the treatment?
Yes, you will need to restrict fluid intake to a minimum of 8 hours prior to the scheduled time of instillation. Also avoid caffiene in the morning prior to the procedure.
Are there any precautions to be taken prior to the procedure?
Do inform your doctor if you are pregnant, nursing a baby, have high fever or blood in urine and if you are on any antibiotics. Also let your doctor know if you have suffered from tuberculosis in the past. This treatment cannot be given in those who don’t have urinary control (total incontinence) and those who are immunocompromised patients.
What exactly is done?
Prior to the instillation you will be advised to pass urine and empty your bladder completely. The BCG is diluted with 40 ml of saline and instilled after inserting a small catheter into your bladder. If during catheterization, there is blood seen, the procedure will be aborted and re-scheduled after a week.
After instillation, the catheter would be removed and you will be told to retain the solution in the bladder for two hours. Till this period of 2 hours you should continue to restrict fluid intake.
At the end of 2 hours, you are advised to sit/squat while urinating to avoid splashing urine on your feet. Inform the nursing staff after you pass urine as they will clean the toilet with bleach. Let the bleach act for 15 minutes before flushing the toilet. Repeat the above process each time you urinate for 6 hours after each treatment even at home. Wash your hands and genital areas thoroughly after you urinate. After you pass urine, you must increase your fluid intake.
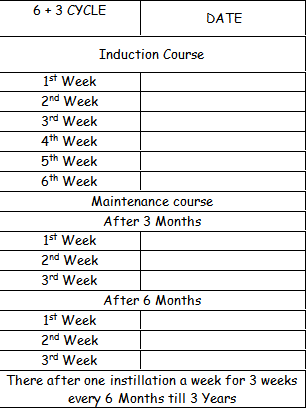
How frequently should I have this treatment?
This will be decided, depending upon the stage and grade of your disease.
Initially you will have one instillation a week for 6 weeks. Then at 3 and 6 months, you will have one instillation a week for 3 weeks. Subsequently you will have one instillation a week for 3 weeks every 6 months for the next 3 years.
What are the side effects of this treatment?
Though BCG is well tolerated, few patients may have low-grade fever (<38.5ºC), burning sensation while you pass urine, frequent desire to pass urine, blood in the urine for a day or two. These can be managed by medications which your doctor will prescribe. But if the above symptoms are severe and persist more than 2 days, you will need to give urine for culture to rule out bacterial infection. However antibiotics like ofloxacin is to be avoided. You may be started on combination oral drug treatment for tuberculosis till the symptoms subside. Subsequent BCG dose may be reduced by your doctor. Some patients may develop high fever (>39.5ºC) or joint pains or skin rash or low blood pressure. You will require admission and emergency management and the antitubercular drugs may need to be taken for 3-6months. Very rarely bladder capacity may shrink after the procedure.
Are there any other special precautions I need to adhere to?
You are advised to abstain from sexual intercourse for 48 hours after every instillation. To use a condom at other times during 6 weeks course of treatment and for six weeks after treatment has ended.
Would I, for any reason need to stop this therapy?
You may be advised to stop this therapy, if you have high fever, burning sensation in urine or if you are passing a lot of blood in urine.
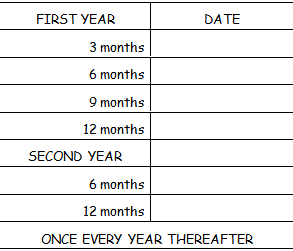
After completing the course of treatment how often should I visit my doctor?
Most patients with cancer of the urinary bladder require regular cystoscopic (Endoscopic) examination of the bladder.
Usually every 3 months for the 1st year
Every 6 months for the 2nd year
And yearly thereafter
CHECK CYSTOSCOPY SCHEDULE:
DIAGNOSIS:
STAGE:
DATE OF INITIAL TUR-BT:
SCHEDULE OF INTRAVESICAL BCG
DIAGNOSIS:
STAGE:
DATE OF INITIAL TUR-BT: